Endoscopy-related infections are among the more frequently reported infections caused by medical devices today. That’s why extensive guidelines are in place for reprocessing reusable endoscopes to limit the risk of infection for patients.
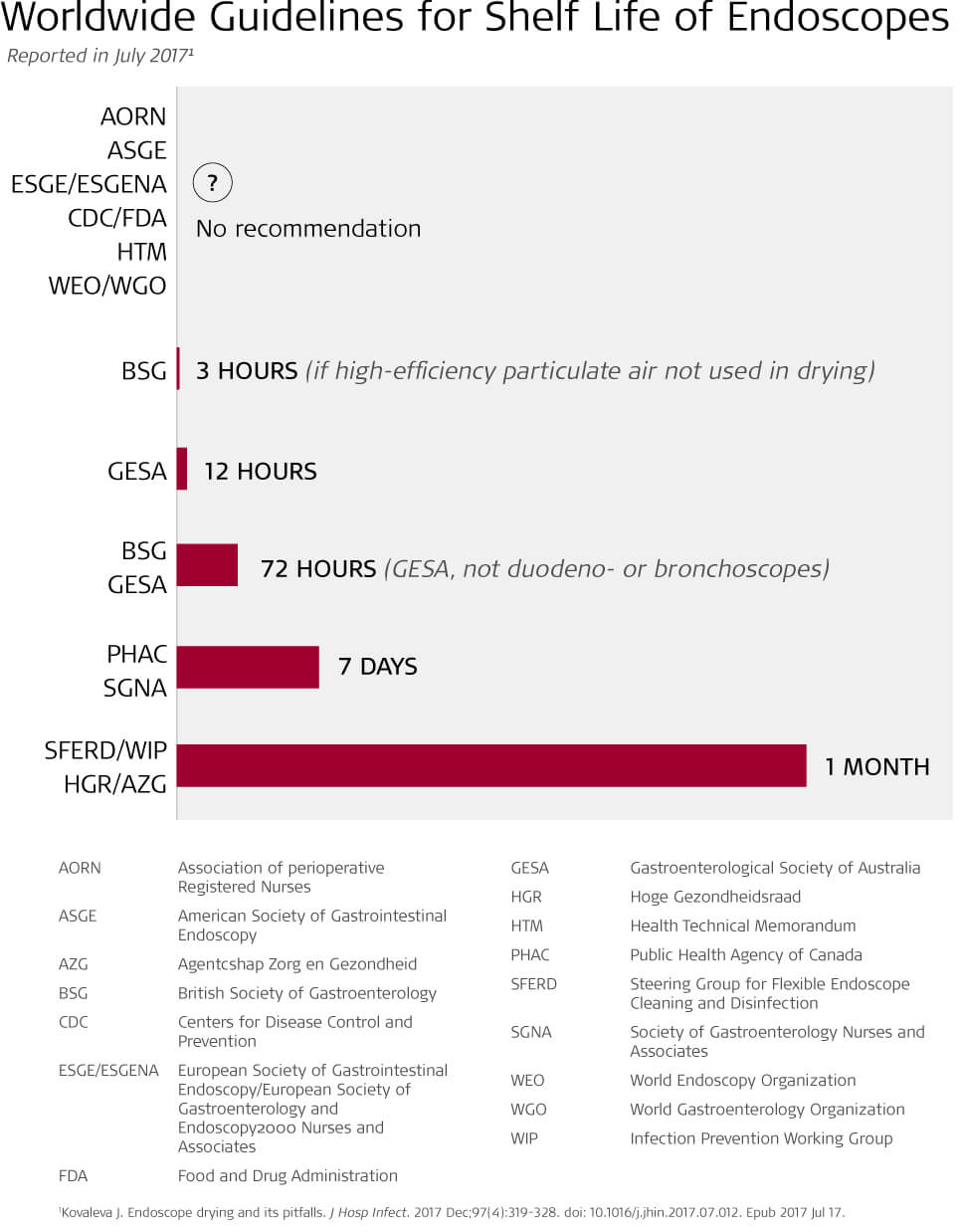
What’s missing from these guidelines, however, are strict recommendations or standard practices for how long endoscopes can safely be stored once they have been reprocessed. This is important given the risk of microbial colonization on the parts of endoscopes that are difficult to clean, sterilize and dry.
A lack of clarity and standardization for endoscope shelf life has sparked some recent studies around the issue, including a trial in which researchers found that endoscopes could hang for 12 weeks in storage cabinets without any trace of microbial growth.
Extending the shelf life of endoscopes can save hospitals valuable time and money. Reprocessing procedures include more than 100 steps and require significant amounts of chemicals and disinfectants. In addition, a longer shelf life could also increase an endoscopy unit’s efficiency as a lack of available endoscopes, because of reprocessing, can cause delays in procedures.
Currently, U.S. multisociety guidelines classify shelf life for endoscopes as an “unresolved issue.” The Healthcare Infection Control Packages Advisory Committee guidelines state the maximum shelf life of endoscopes has not been determined and note the length of time may vary on the organization, how the scope is stored, and the manufacturer’s instructions for use. The Association of periOperative Registered Nurses previously recommended an endoscope shelf life of only five days, but in 2016, revised their recommendations to between 48 hours and 56 days.
As no consensus exists, some recent studies have sought to determine exactly how long endoscopes can be stored before there’s risk of microbial growth. The results have pointed less to length of time an endoscope is stored and more to the effectiveness of endoscope cleaning.
A study published in the Gastrointestinal Endoscopy Journal in 2015 concluded endoscopes could be stored for as long as 21 days without developing a harmful amount microbial growth. Researchers did find 33 positive cultures on the observed scopes, but 29 of those cultures were determined to be “typical skin or environmental contaminants, thus clinically insignificant.” Other contaminants were found to not be dangerous due to their small amount.
Based on their findings, researchers determined shelf life for endoscopes could extend to 21 days.
The study’s authors—Andrew S. Brock, MD; Lisa L. Seet, PhD; Janice Freeman, RN, CCRC; Bernadette Garry, RN; Phyllis Malpas, RN, CGRN; and Peter Cotton, MD—also noted:
“It should be emphasized that our conclusions and recommendations clearly apply only when endoscopes are reprocessed and stored in optimal fashion. It is conceivable that short shelf times may occasionally have minimized the potential adverse effects of inadequate reprocessing at some centers and that lengthening the time in such circumstances could be detrimental.”
Similarly, a 2019 report published by the Society of Gastroenterology Nurses and Associates concluded that time in storage did not contribute to microbial growth on an endoscope. Inadequate cleaning and drying were to blame for contamination. The study, “Evaluation of 12-Week Shelf Life of Patient-Ready Endoscopes,” also noted that improper handling of scopes while in storage could inadvertently contribute to contamination of cleaned scopes.
The authors of the study—Valerie Lacey, RN, BSN, CGRN; Karron Good, RN, ADN, CGRN; Chris Toliver, RN, ADN; Shirley Jenkins, MT (ASCP); Pamela B. DeGuzman, PhD—noted it is not universally required for staff to wear personal protective equipment when accessing storage cabinets. This lack of protocol can have significant impacts on the cleanliness of scopes in storage.
In their study, four colonoscopes and two gastroscopes were reprocessed, sterilized, flushed with alcohol and then air dried with medical-grade air prior to being hung in a storage cabinet with a solid vented door for 12 weeks. Throughout the allotted time, any staff accessing the storage cabinet for other scopes wore personal protective equipment.
The authors concluded that limiting endoscope shelf life may have no effect on contamination so long as there was consistent use of personal protective equipment when accessing storage cabinets and the scopes were cleaned and disinfected adequately prior to storage.
Both studies were limited by small sample sizes and both were conducted using scopes at a single location. Neither study included bronchoscopes, which have been connected to higher numbers of endoscopy-related infections than colonoscopes and duodenoscopes.
Reprocessing endoscopes is a lengthy and complicated procedure, and the final steps are just as important as the first steps in guaranteeing patient safety.