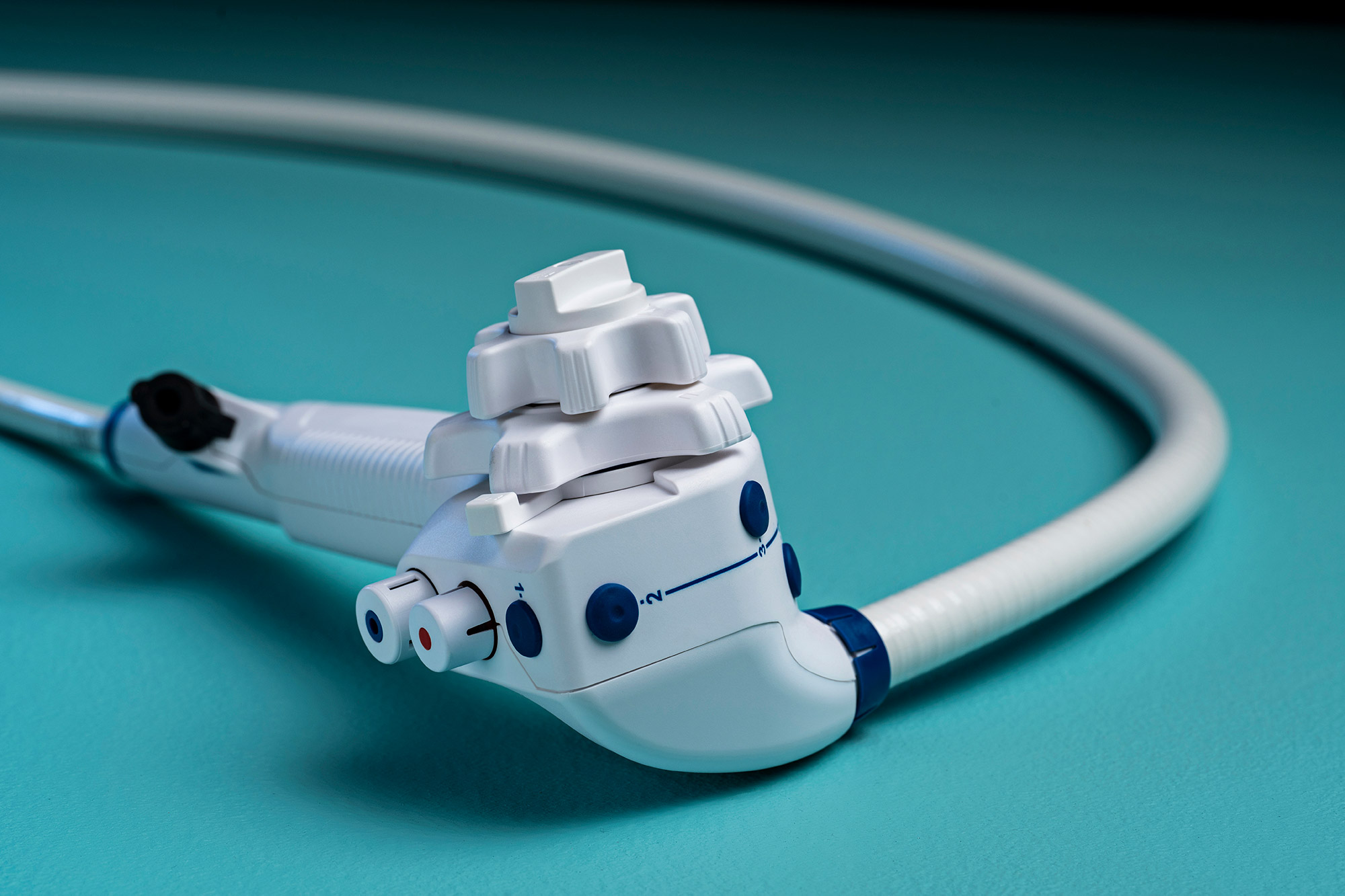
Ambu has received 510(k) regulatory clearance from the U.S. Food and Drug Administration (FDA) for its aScope Gastro Large — the world’s first single-use therapeutic gastroscope.
The novel device features a 4.2mm working channel and expands Ambu's portfolio of single-use endoscopes for upper gastrointestinal (GI) procedures. The clearance enables physicians to address a wider range of clinical needs across intensive care units (ICUs), operating rooms (ORs), and endoscopy suites.
"Our therapeutic gastroscope solution is a vital step forward in our gastroenterology journey," Britt Meelby Jensen, Ambu’s CEO, said in a news release. "Not only does it expand and strengthen our gastroscope portfolio, empowering U.S. physicians with two complementary high-performance solutions for upper GI procedures targeting diverse clinical needs, but it also marks an important way forward for hospitals and GI professionals wishing to alleviate the strain of reprocessing, optimize workflow, and bring faster care to patients."
The aScope Gastro Large addresses acute therapeutic procedures in the ICU, such as bleeding management and food impaction, as well as more complex endoscopic procedures in the endoscopy unit, including direct endoscopic necrosectomy and stenting.
Compared to currently available 3.7mm therapeutic gastroscopes, the aScope Gastro Large offers significantly higher suction performance, both with and without tools, making it a valuable device for managing acute upper GI bleeding.
Not only is it the first single-use therapeutic gastroscope with a 4.2 mm working channel, the aScope Gastro Large is also the first endoscope made with bioplastic materials. The bioplastic handle is derived from a mix of fossil-based and second-generation feedstock, such as recycled food waste, reducing the device's carbon footprint.
The FDA clearance follows European regulatory approval (CE mark) in September 2023.
Research finds Ambu’s single-use gastroscope portfolio to offer high completion and satisfaction rates for EGD, capable of evaluating and treating upper GI bleeding, and to be effective in advanced therapeutic procedures.