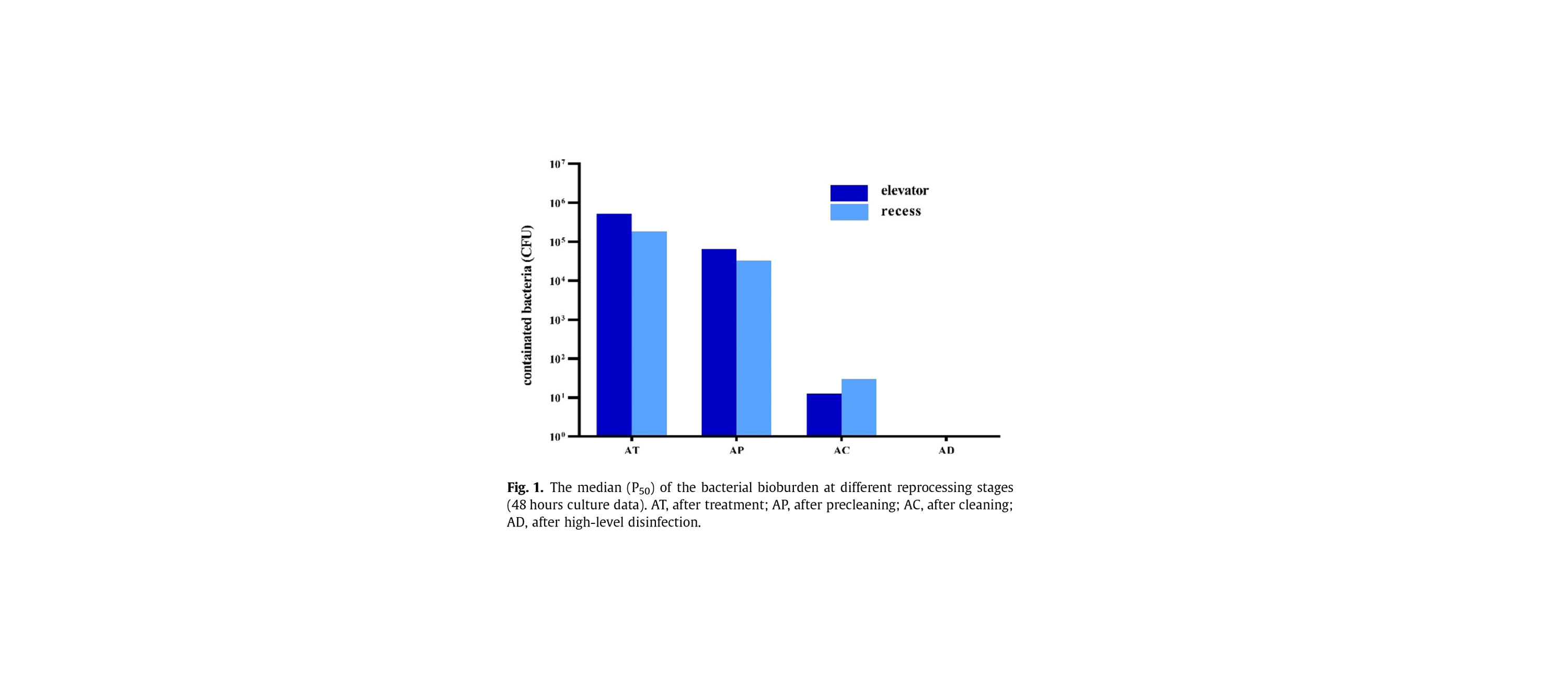
Even after bedside cleaning, manual cleaning, and high-level disinfection (HLD), contamination may remain present on the elevator mechanism of reusable duodenoscopes.
In fact, researchers who cultured such devices at a tertiary hospital in China which performs approximately 2,400 endoscopic retrograde cholangiopancreatography (ERCP) procedures annually found just over 7 percent of bacterial cultures remained positive after the process.
They summarized their findings in the American Journal of Infection Control.
“New staff members should undergo rigorous professional training, and the daily cleaning quality needs constant surveillance,” the authors write. “In addition, initiatives should focus on the development of specialized cleaning tools specifically for the elevator mechanism or even the advent of fully automatic cleaning and disinfection devices to reduce human involvement.”
They add that evidence shows meticulous manual cleaning allows for superior HLD.
Novel single-use duodenoscopes — such as those manufactured by Ambu A/S — are fully disposable, eliminating the need for these steps as scopes are discarded after each use.
The study involved 112 microbial samples collected from six reusable duodenoscopes ranging from six months to three and a half years in use. Duodenoscopes pose reprocessing challenges because of their “intricate structures,” according to the authors. The elevator mechanism at the distal end has drawn scrutiny in recent reports about endoscopy-related infections, they note.
“Although many studies affirm that even with strict compliance to technical guidelines and manufacturer-recommended reprocessing procedures, the sophisticated design of duodenoscopes might still harbor harmful pathogens, including drug-resistant bacteria,” the authors write.
Contributing factors to infection transmission, the authors add, include delayed or insufficient post-procedure cleaning, small crevices on the elevator surface, and incorrect positioning during automated reprocessing.


