The Centers for Disease Control and Prevention estimates that more than 2 million people in the U.S. are infected with antibiotic-resistant organisms every year, causing 23,000 untimely deaths. These infections also carry a big price tag, estimated to be about $20 billion in excess health care spending each year.
Antimicrobial resistance (AMR), defined as the ability of bacteria, fungi and parasites to develop a resistance to antimicrobial treatment, is one of the biggest health threats facing the world today. It is estimated that AMR causes approximately 10 million deaths globally per year by 2050.
The primary culprit behind the rapid spread of global AMR has been the unnecessary and inappropriate use of antibiotics for decades. When penicillin was discovered by Alexander Fleming in 1928, he warned of the potential for bacteria to be able to adapt and resist the drug and others like it. AMR was going to happen naturally, but the more antibiotics were introduced, the faster resistance would grow.
Despite Fleming’s warnings, as early as 1968, the Society for Healthcare Epidemiology in America (SHEA) reported that as many as half of the antibiotics prescribed in the U.S. were not medically necessary.
The threat of AMR poses different challenges and has varying costs around the world. In low- and middle-income countries, the poor are severely lacking access to lifesaving antibiotics while the middle classes are often overconsuming the drugs and speeding the spread of AMR. Around the world, many life-saving medical procedures are at a risk of some day failing due to AMR, including joint replacements, cesarean sections, and chemotherapy.
The potential costs of AMR are “incredibly uncertain and potentially catastrophic,” according to a 2019 report, “The challenge of antimicrobial resistance: What economics can contribute.” The authors -- Laurence S. J. Roope, Richard D. Smith, Koen B. Pouwels, James Buchanan, Lucy Abel, Peter Eibich Christopher C. Butler, Pui San Tan, A. Sarah Walker, Julie V. Robotham, and Sarah Wordsworth – also concluded that estimates of the total cost of AMR “are fraught with uncertainty, and there is a risk of the cost being far higher than current best estimates.”
The global costs associated with AMR are so varied because the potential direct and indirect consequences are difficult to quantify. AMR can cause treatment failures, longer hospital stays, increased risk of chronic illness, and ultimately, death. AMR can also require a need for more antibiotics to be used if the first round fails due to resistance.
Treating infections caused by antimicrobial-resistant bacteria is by nature more expensive to treat than non-resistant infections. According to the World Health Organization, this is because the illness usually lasts longer and requires more expensive drugs.
A review by Roope and his fellow authors of papers that have tried to estimate the costs of AMR per patient episode showed they varied from less than $5 to more than $55,000. Yet the papers estimated only the direct health care costs, such as additional hospitalizations.
Boiling down the cost of AMR to specific types of procedures is also difficult to quantify. For example, hospital-acquired tuberculosis from an endoscopy, of which there were 9,000 reported cases in 2017, can cost from $18,000 to $500,000. Costs will be on the higher end of that spectrum if the drug-resistant form of the disease is being treated.
While not all hospital-acquired infections are caused by antimicrobial-resistant bacteria, many are and they require intense antibiotic treatment. And more hospital-acquired infections have been linked to contaminated endoscopes than any other medical device.
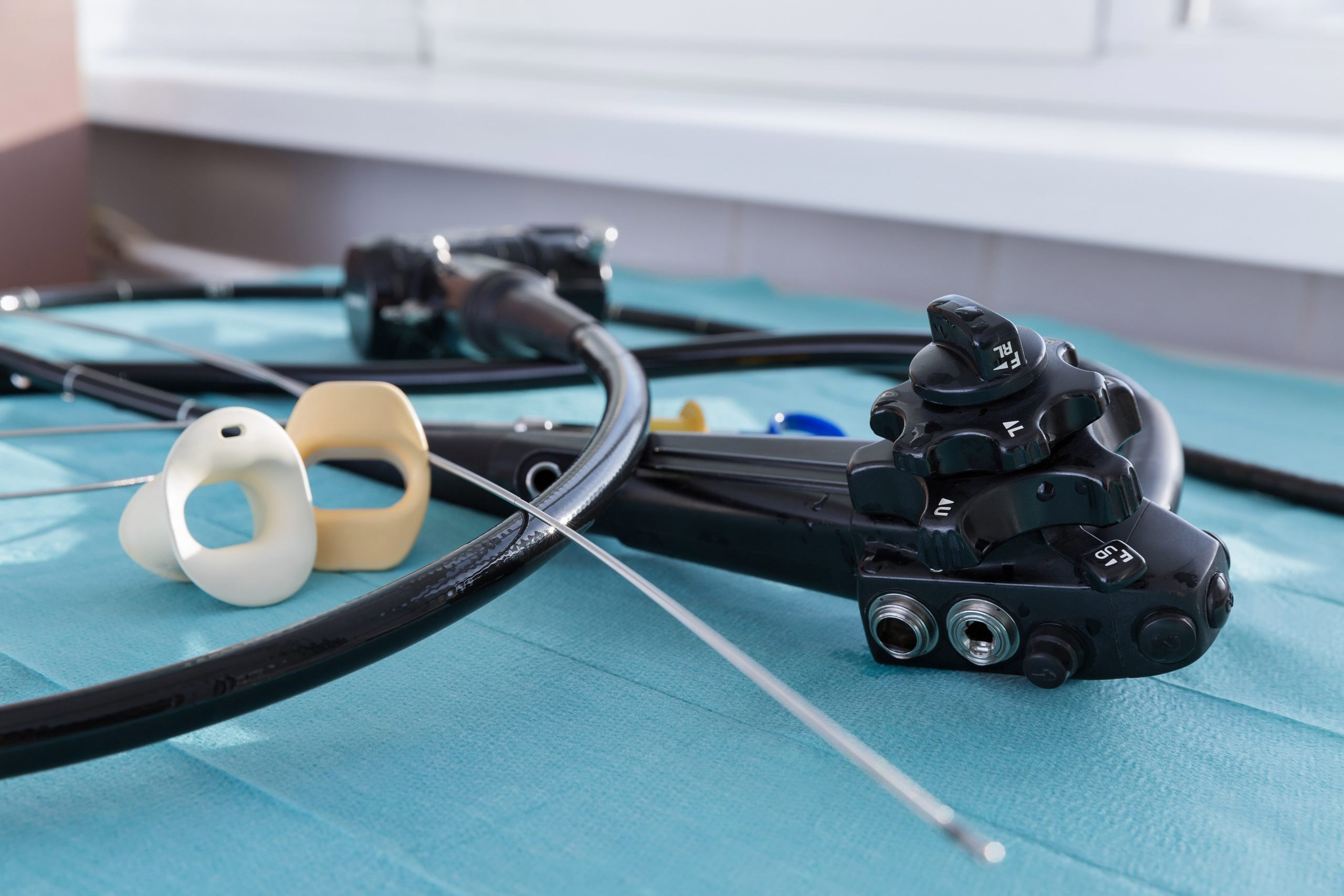
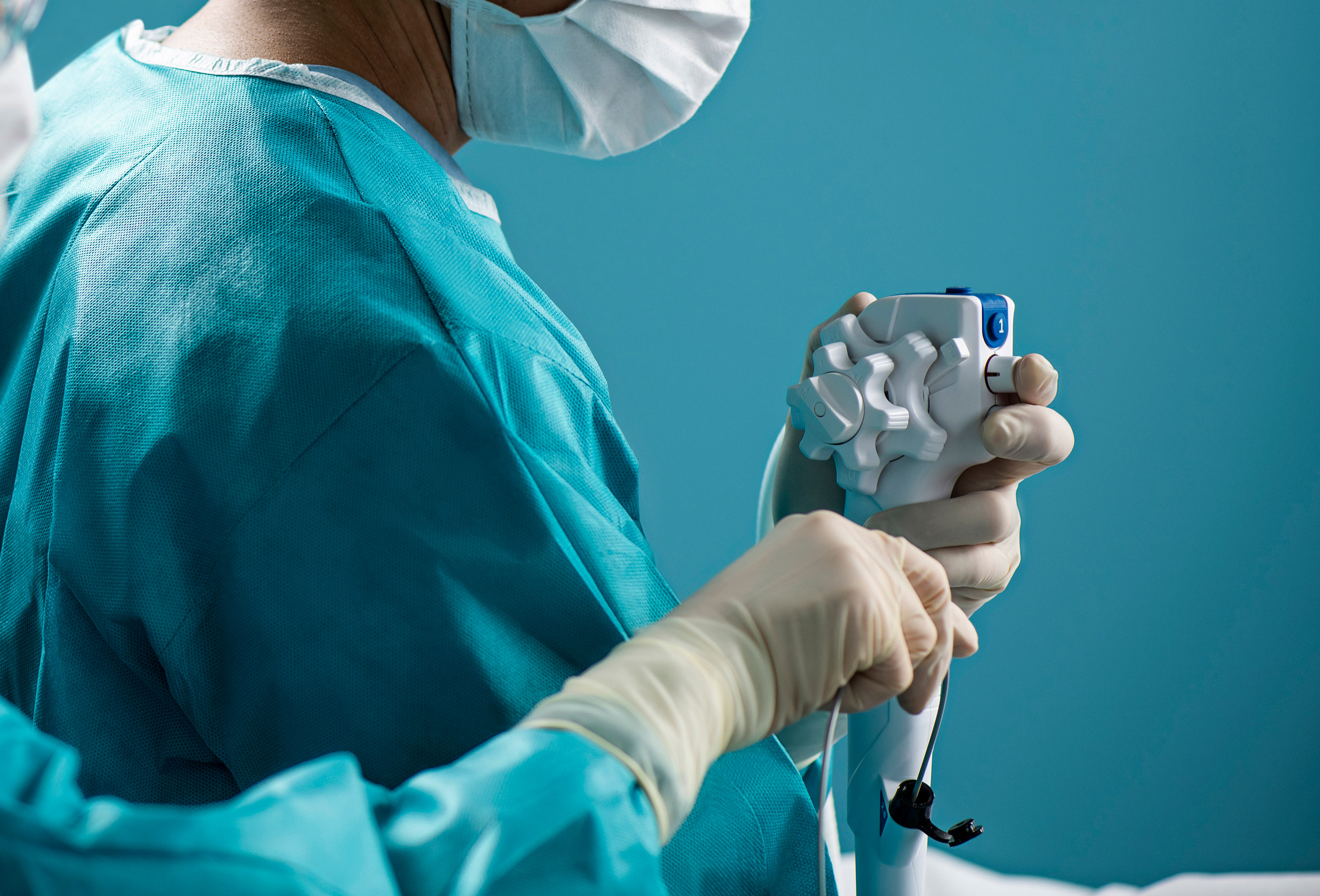
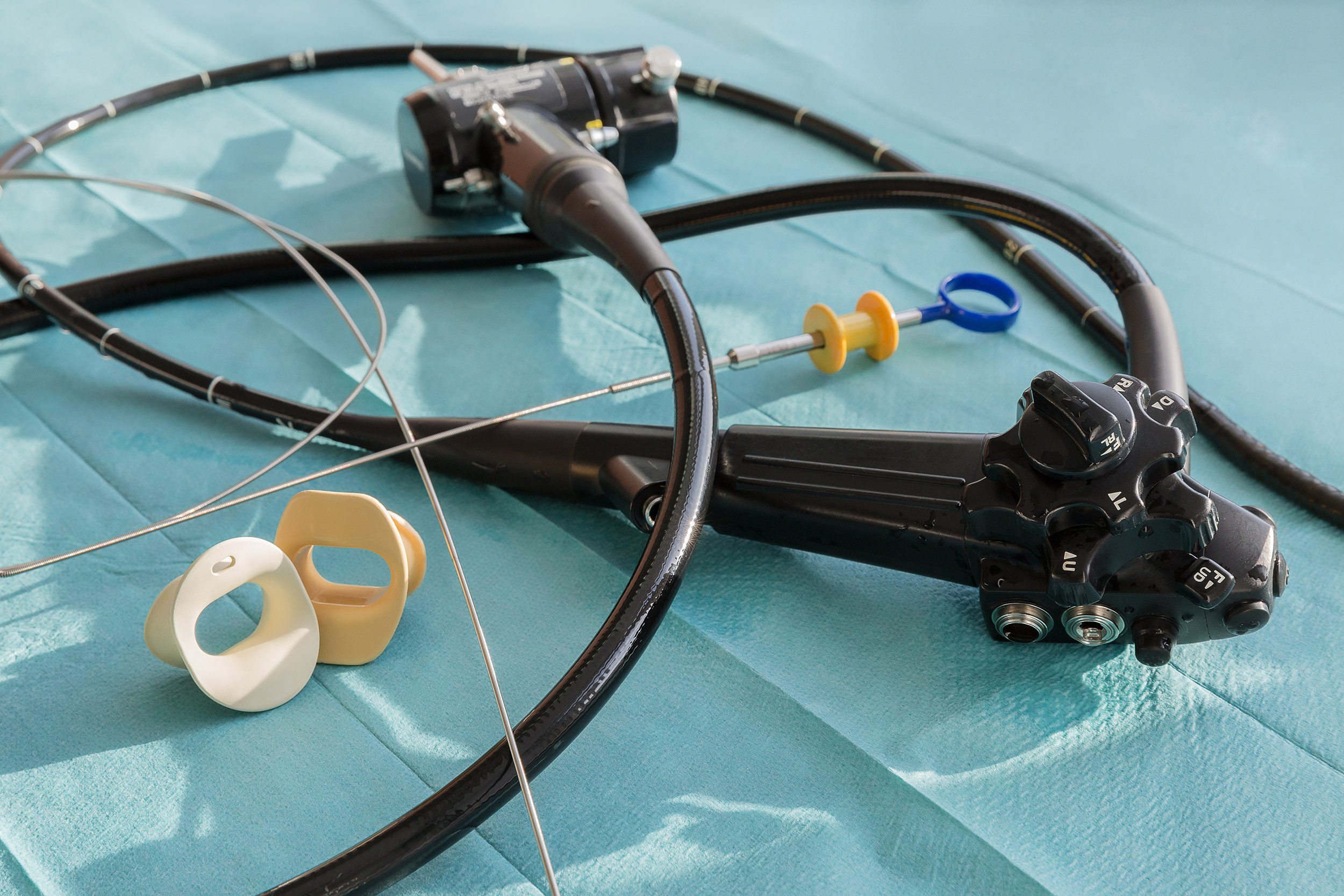
Endoscopes are long, delicate instruments used to provide visual access to a hollow organ or body cavity. The devices are used for medical assessment and diagnosis, for collecting tissue or secretions or for administering treatments – and they enable physicians to perform these vital tasks without having to perform surgery, which can pose a greater risk to the patient.
Flexible endoscopes can harbor infectious bacteria because they are difficult to clean and disinfect. Reprocessing is a long and arduous process, and research has shown that even if all industry guidelines are followed, there is still a lingering risk of contamination. In addition, endoscope damage has been linked to device-related infections.
Sepsis, ventilator-associated pneumonia, and tuberculosis are some of the infections that have resulted from contact with flexible bronchoscopes. Costs associated with sepsis can be particularly high because 62 percent of those hospitalized with sepsis are re-hospitalized within 30 days.
From 2012 to the spring of 2015, 250 life-threatening infections worldwide were linked back to contaminated endoscopes. Included on that list were infections caused by Carbapenem-resistant Enterobacteriaceae, which the CDC refers to as a “nightmare bacteria” given its resistance to almost every form of antibiotic.
One of the most common organisms found in contaminated endoscopes is P. aeruginosa. This organism accounts for 9 percent of all hospital-acquired infections in the U.S and is the second most common cause for nosocomial pneumonia, health care-associated pneumonia, and ventilator-associated pneumonia. Infections caused by this bacterium are also incredibly difficult to treat, due to the ability of P. aeruginosa to adapt very quickly to resist antibiotics.
AMR needs to be addressed globally for the threat to decrease. Even so, antimicrobial stewardship programs in the U.S. can still make an impact combating AMR and help lower the associated treatment costs. Such coordinated efforts can improve antibiotic use with the ultimate goal of increasing patient outcomes and reducing unnecessary costs. St. Tammany Parish Hospital in Covington, Louisiana, implemented an antimicrobial stewardship program and saw $1.3 million in savings in just the first 18 months of the program, as reported by the Pew Charitable Trusts in 2016.
Due to the associated costs with device-related infections, should comprehensive antimicrobial stewardship programs include proper infection prevention protocols for handling and using devices like endoscopes? Reducing hospital-acquired infections from devices, like endoscopes, will help reduce the need for additional antibiotic treatment as well as an increased spread of antibiotic-resistant bacteria.
One way to eliminate the risk of device-related infection from endoscopes is to use sterile, single-use instruments. Disposable, single-use bronchoscopes bypass the risk of infection seen with reusable endoscopes since there is no chance of bacterial transfer from patient to patient. And a single-use bronchoscope costs between $220 and $315, while reusable bronchoscopes could run a tab of $281 to $803 per procedure when considering reprocessing, repair, maintenance, and risk for treatment-related costs due to infection.
Visit the Ambu website to find out more about single-use, sterile out of the package bronchoscopes and rhinolaryngoscopes. The economic impact of these devices is similar to reusable devices, according to a number of cost analysis studies.