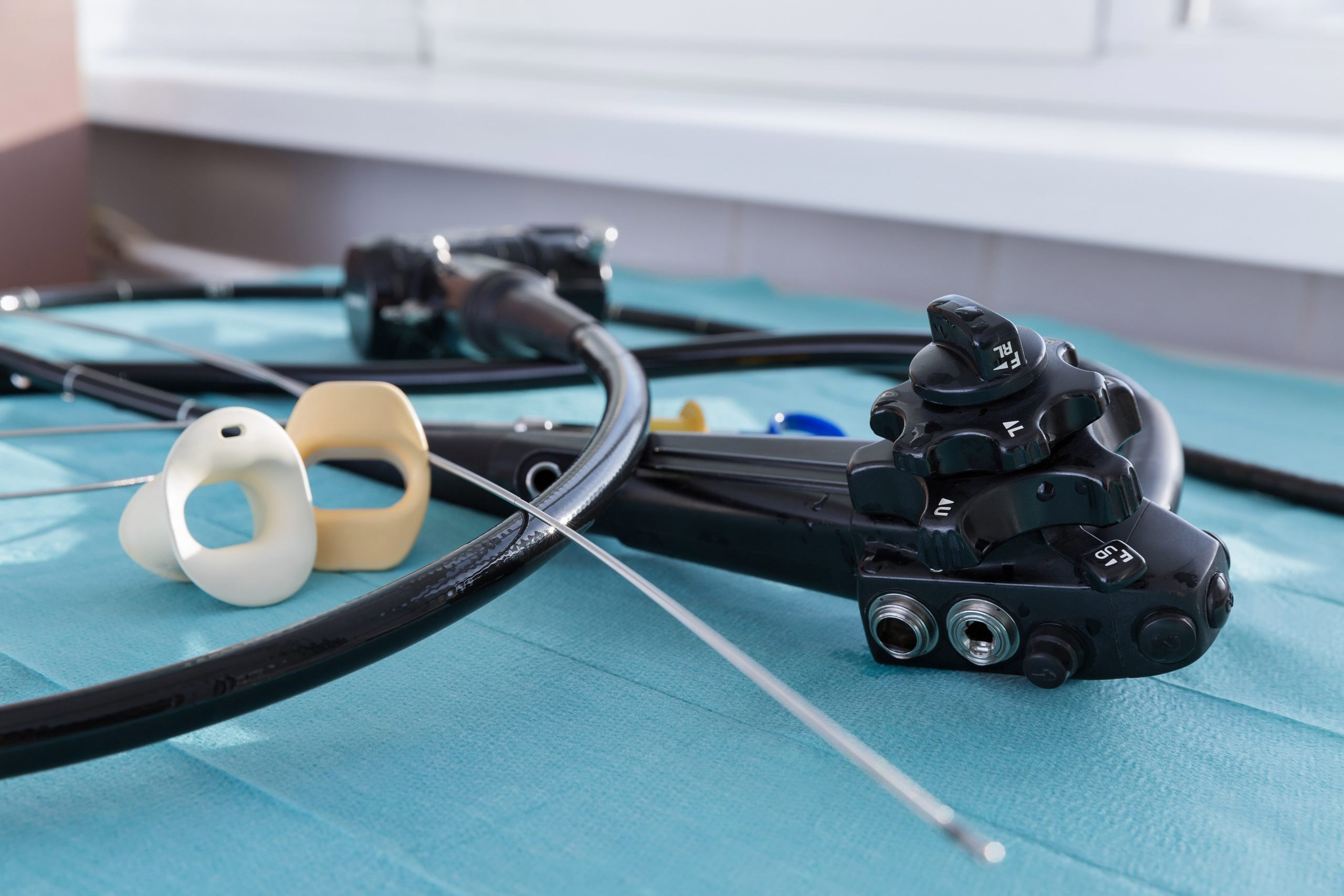
When infection preventionists (IPs) from Northwestern Medicine investigated the high-level disinfection (HLD) processes of a urology practice in July 2021, they identified several “breaches” in infection control practices.
Staff members responsible for HLD processing had been using expired chemical test strips and indicators during the reprocessing of reusable cystoscopes and prostate biopsy probes, according to a report published in the American Journal of Infection Control.
Infection preventionists attributed several security breakdowns in infection control practices to a lack of education and understanding of high-level disinfection practices.
The breaches included gaps in staff training, a lack of supplies, and inadequate staff supervision.
As part of their investigation, the IPs examined patient records from July 2019 through July 2021 to determine if any patients had a history of exposure to bloodborne pathogens, urinary tract infections or positive urine culture test results.
The report documented several cases of urinary tract infections following cystoscopy procedures linked to questionable HLD processes.
The IPs recommended that all cystoscopy procedures be suspended at the outpatient urology practice until the security breaches had been resolved.
The IPs also highlight the importance of ongoing leadership and infection prevention oversight in their report — HLD is a highly complex task requiring supervision to ensure patient and staff safety, they add.
A new, highly anticipated update to standards for reprocessing reusable endoscopic medical devices classifies flexible endoscopes — including cystoscopes and ureteroscopes — as "high-risk," necessitating sterilization rather than high-level disinfection to rid the scopes of contaminants that could harm patients.
The document, ANSI/AAMI ST91:2021, Flexible and semi-rigid endoscope processing in health care facilities, has been the subject of much debate in clinical and infection prevention circles since its release. It states that high-level disinfection (HLD) may not “reliably inactivate” certain types of microorganisms.
All flexible endoscopes should undergo sterilization to lower the risk of biofilm formation and enhance patient safety, according to the updated guidance from the Association for the Advancement of Medical Instrumentation (AAMI). Cleaning verification tests should be performed after each use of these scopes.