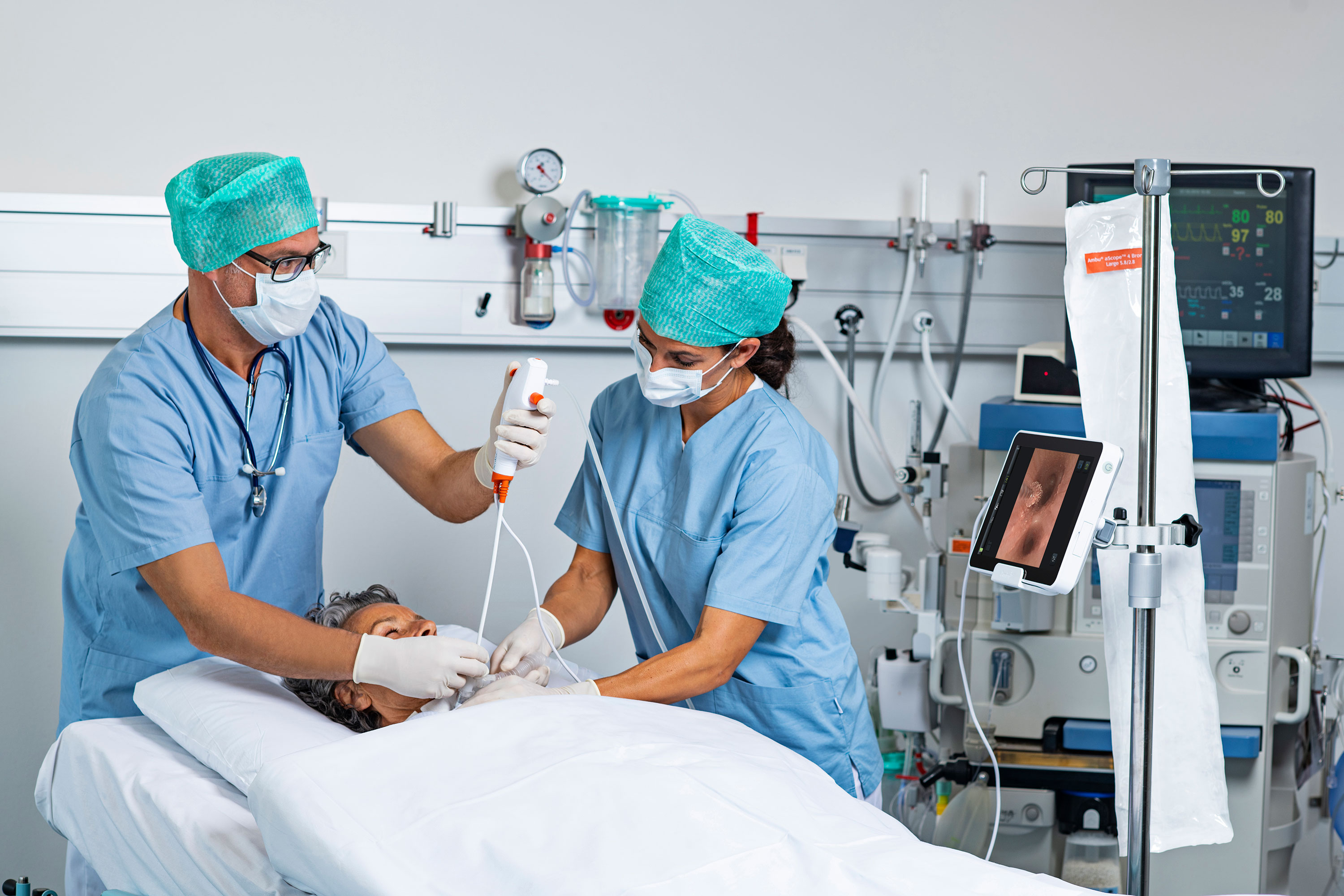
Two “inherent themes” persist throughout the recent update to a high-profile standard for reprocessing reusable endoscopes, according to an expert in infection prevention and control, infectious disease, and patient safety.
The standard — ANSI/AAMI ST91:2021, Flexible and semi-rigid endoscope processing in health care facilities — refers continuously to manufacturers’ instructions for use and frequently defers back to institutional policy, according to Dr. Hudson Garrett Jr. in a recent webinar hosted by Ambu A/S.
And that’s significant because “you’re going to find some things that are pretty clear and there are some things that, in my opinion, are not,” he says of the ST91 update.
In the webinar, Garrett — an Ambu consultant — explores what can be found in the document and what was excluded. He also examines what led a group of GI societies to issue a joint rebuttal of ST91 — a surprising response, he says of the joint statement.
In addition to the steps facilities must take to comply with ST91, Garrett reviews recent FDA-issued safety communications involving reusable bronchoscopes, duodenoscopes, and urological endoscopes.
All three say to eliminate reprocessing when possible, which can be done through a transition to single-use endoscopes. He also discusses an urgent recall of reusable urological endoscopes.
Garrett is an adjunct assistant professor of medicine in the Division of Infectious Diseases at the University of Louisville School of Medicine. He also is co-founder of the nonprofit Infection Prevention Institute which disseminates best practices of infection prevention and control across healthcare globally.
Watch the entire webinar below: