More than 4,000 veterans who received endoscopies or other procedures at the Carl Vinson VA Medical Center in Dublin, Georgia, were informed that they may have been exposed to infection due to irregularities in equipment reprocessing.
Although the risk of infection was determined to be “very low” following an internal review, all veterans potentially impacted were notified, according to a letter issued by the medical center earlier this month.
Anyone who had a dentistry, endoscopy, urology, podiatry, optometry or surgical procedure during the period between January 1, 2021, through January 27, 2022, was sent a letter.
“For your own reassurance … you may wish to be tested for infections like hepatitis B, hepatitis C and HIV,’’ the hospital’s director, Manuel M. Davila, wrote in the letter.
The testing for those blood-borne pathogens is not required, but the hospital is offering it free to veterans who choose to participate. It is being recommended even for people not showing symptoms.
If any veterans test positive, the VA will help family members and caregivers to arrange treatment, the hospital said.
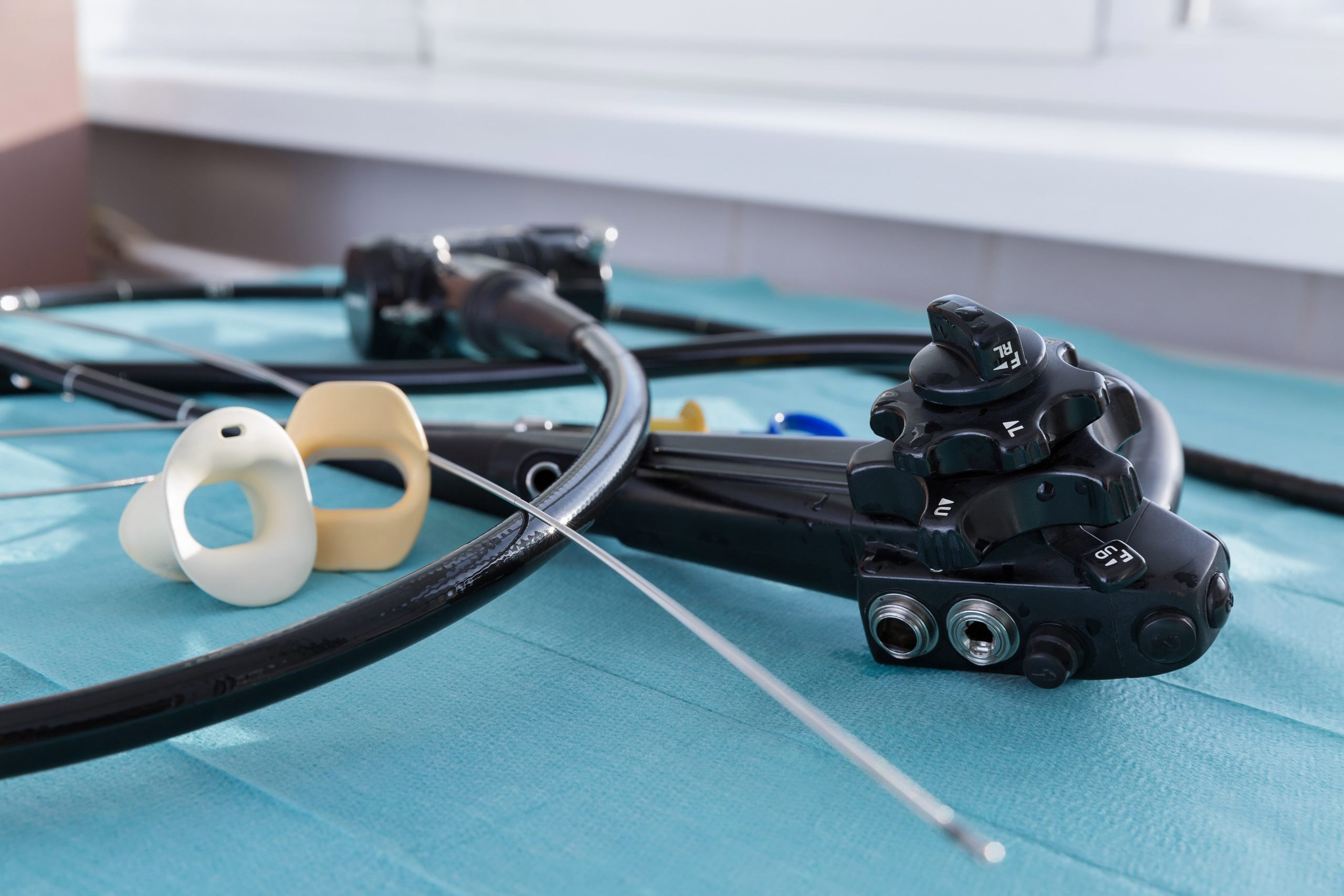
The hospital’s reusable equipment typically goes through a multi-step process between patients, to ensure proper cleaning or sterilization, Davila wrote. Hospital administrators learned during an internal review, however, that “there may have been times when all the steps necessary for complete and safe cleaning or sterilization were not followed.”
Procedures were halted from Jan. 12-14, during which time all equipment was reprocessed and staff retrained to make sure they understand current manufacturer guidelines.
"If we find information or we find concerns, then we may look back further than January,” Davila told local CBS affiliate WMAZ. “We may go back to two years, or we may go back three years. It just depends what we find in the initial look back."
Findings from that review are expected by Feb. 24.
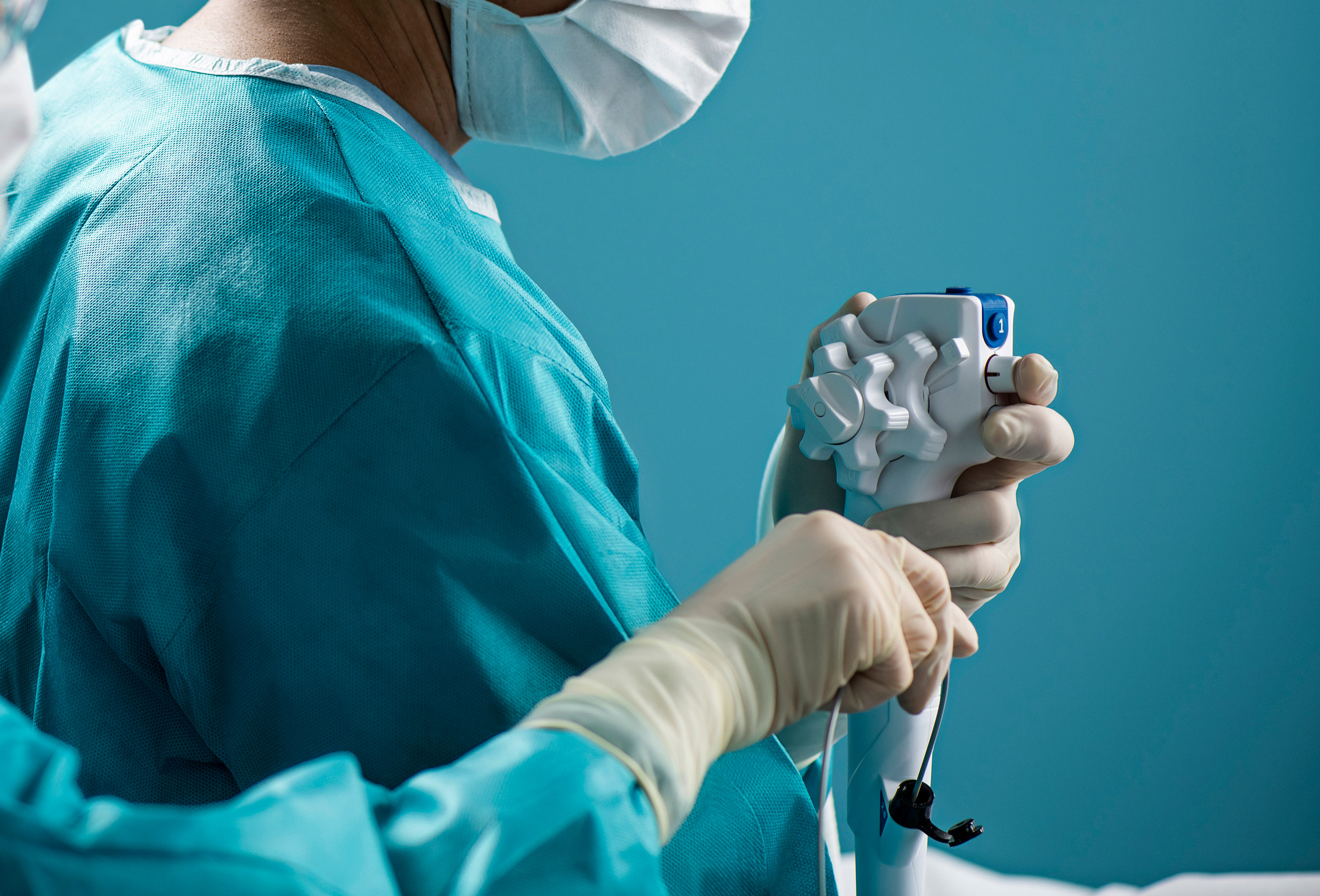
Many reusable endoscopes are notoriously hard to clean, requiring extensive reprocessing that involves more than 100 steps and two or more hours to complete. Cross-contamination from patient-ready endoscopes, while rare, is a concern because of the complex processes in cleaning the scopes and the potential for human error.
Medical device companies such as Ambu A/S and Boston Scientific Corp. are developing a range of endoscopes that can be used once and discarded, or have disposable components, to prevent cross-contamination and boost patient safety.
The fully single-use devices never need reprocessing or repairs and recent regulatory moves have accelerated a conversion to single-use.
In August 2020, the U.S. Food and Drug Administration (FDA) updated its recommendation that duodenoscope manufacturers and healthcare facilities transition to duodenoscopes with disposable components and scopes that are fully disposable.
In April 2021, the FDA announced it was investigating “numerous” medical device reports (MDRs) describing patient infections and other possible contamination possibly associated with reprocessed urological endoscopes.
The U.S. Food and Drug Administration (FDA) issued a safety communication update in June 2021 recommending that healthcare providers consider using single-use bronchoscopes when there is increased risk of spreading infection and when treating COVID-19 patients.
The Department of Veterans Affairs has had a number of high-profile cases involving medical equipment that wasn’t properly cleaned. Between 2003 and 2008 in Murfreesboro, Tennessee; in 2008 in Augusta, Georgia; and from 2004 to 2009 in Miami, 11,000 veterans underwent endoscopic procedures with unsterilized equipment, placing them at risk for cross-contamination, according to Military.com.