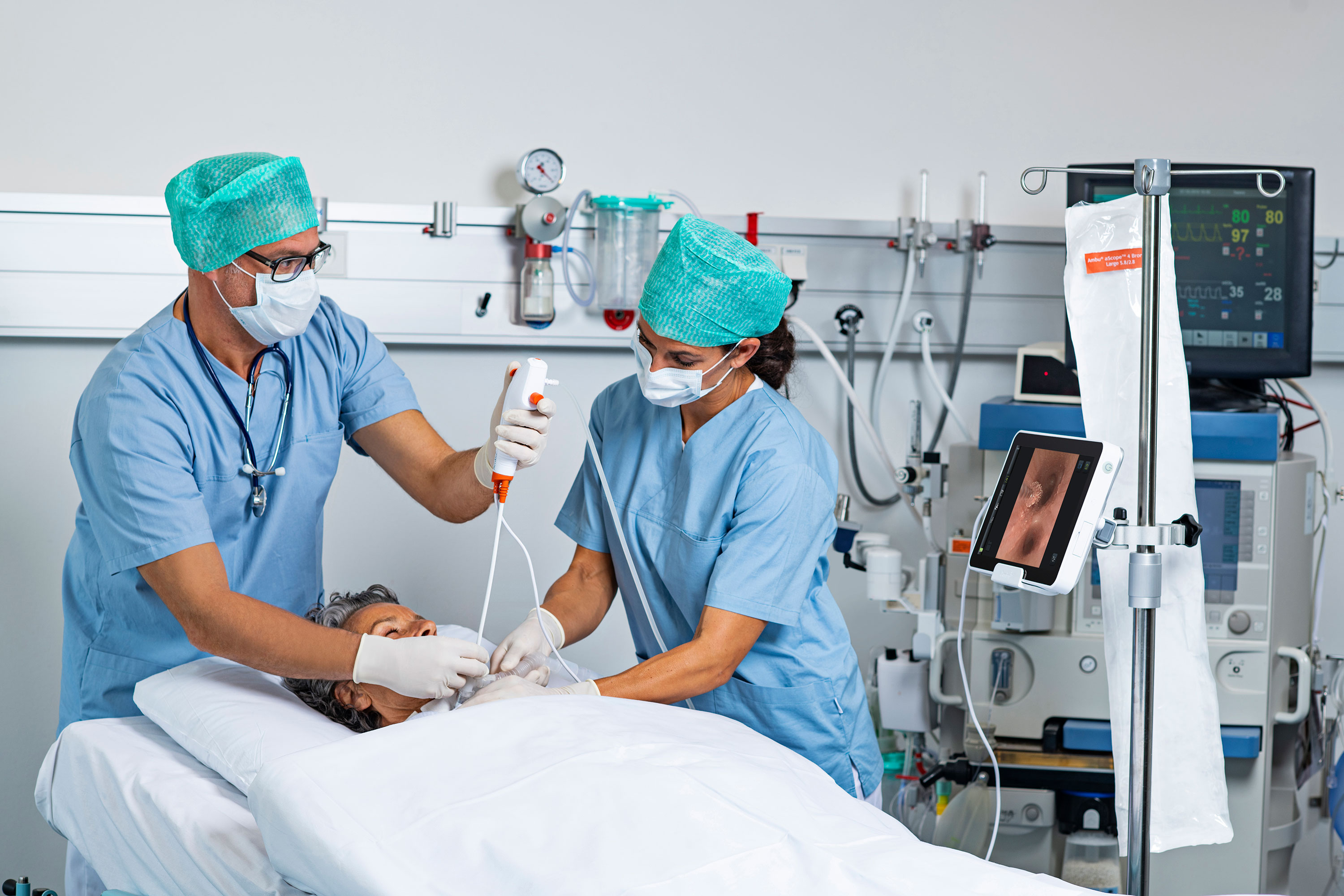
The single-use vs. reusable endoscope debate has evolved in recent years from one of cost, to performance and now a broader conversation about risk management — what can be done that’s in the best interest of the patient.
So says Dr. Hudson Garrett Jr. in a recent webinar, “Risks Between Reusable vs. Disposable Flexible Endoscopes: A Clinical Risk Management Perspective.” The webinar was sponsored by Ambu Inc.
The COVID-19 global pandemic put the power of single-use endoscopes to keep patients and healthcare workers safe on full display. At the same time, rapid advancements in single-use technology have made more complex procedures possible, and new analyses show the benefits that single-use scopes can offer by eliminating large amounts of reprocessing wastewater and the required personal protective equipment (PPE).
Although infection by cross-contamination remains rare, patients are increasingly aware that they are possible and have begun requesting single-use devices, Garrett said. Hospitals and doctors recognize that reprocessing is no guarantee that scopes will truly be patient-ready, despite the best efforts of reprocessing staff.
And just because there have not been widespread reports of infections, that does not mean they are not occurring.
“The absence of outbreak data is not the absence of an outbreak,” Garrett said. “If we’re not being proactive in our surveillance efforts, and we’re not really looking for this information, it’s very, very difficult for us to realize the scope of the issue.”
Garrett is president and CEO of Community Health Associates LLC in Atlanta, co-founder of the nonprofit Infection Prevention Institute and adjunct assistant professor of medicine in the division of infectious diseases at the University of Louisville School of Medicine. He's also an Ambu consultant.
Endoscope designs make it easy for organisms to grow and for water to get trapped inside. Steps can sometimes be missed in the rush of getting scopes ready for the next patient, and recontamination can still occur even after all the steps are followed perfectly.
“This is not the fault of reprocessing technicians, it’s not the fault of nurses. It’s the fault of the system that we have designed in order to handle this process,” Garrett said. “We can either stay status quo … or we can do something to change it.”
Design, reprocessing, complexity of devices and time constraints are all factors to consider when evaluating reusable vs. single-use scopes.
In May 2021, the FDA announced an update to its Section 522 guidance to address public health questions on a large number of adverse reports submitted for gastrointestinal devices already approved and on the market — what became known in the industry as the 522 Study.
The study looked at whether properly trained reprocessing personnel could consistently clean duodenoscopes by following manufacturing instructions and whether microbial contamination remained after reprocessing. FDA safety alerts soon followed in GI, bronchoscopy and urology.
Still, those safety alerts only go so far.
“FDA safety alerts, while they sound really bold, and great, and pretty aggressive, they don’t have a lot of teeth from a legal and a regulatory and a risk management standpoint,” Garrett says. “They don’t say, 'Thou must' ... .”