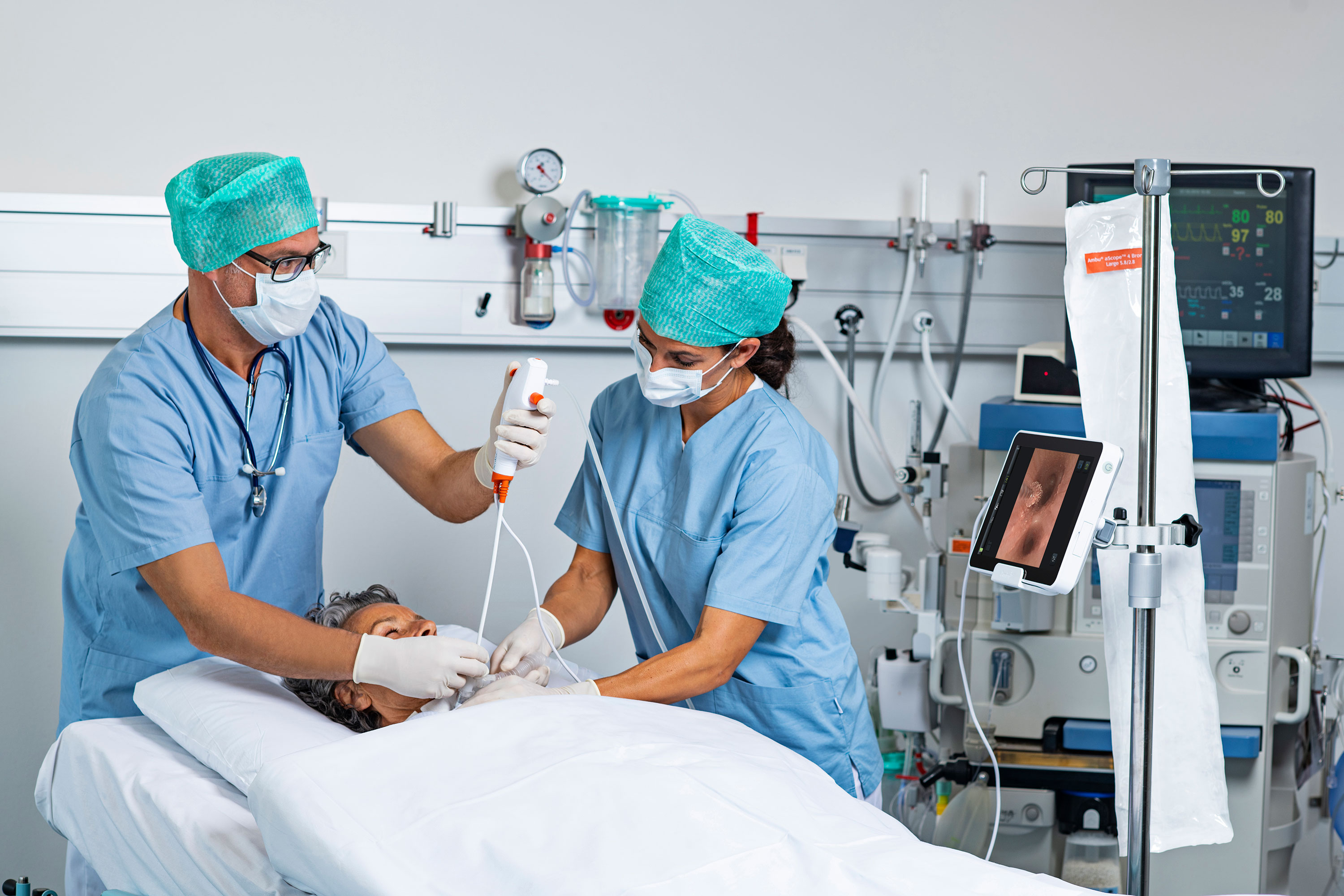
Flexible endoscope reprocessing has come under increased scrutiny as the U.S. Food and Drug Administration continues to investigate new medical device reports related to possible cross-contamination from these devices.
In the last 18 months, the agency has put out new or updated safety communications around three types of flexible endoscopes – duodenoscopes used for endoscopic retrograde cholangiopancreatography (ERCP), urological endoscopes (including cystoscopes and ureteroscopes), and flexible bronchoscopes.
The latest of these communications from the FDA recommended that providers shift to sterilization of flexible bronchoscopes during reprocessing. And in the wake of COVID-19 and awareness of other highly transmissible diseases, the FDA also now recommends providers use single-use bronchoscopes in cases of high risk for infection transmission.
So, what are the hot topics in endoscope reprocessing in the wake of all this activity?
A recent article from the Gastroenterology & Endoscopy News Priority Report highlights a few as discussed by Dr. Michelle Alfa, a microbiologist who has done extensive research on endoscope reprocessing, and Dr. Gregory Cote, a professor of gastroenterology and hepatology at the University of South Carolina.
Storage Cabinets
A 2018 survey of duodenoscope reprocessing practice patterns showed that forced air drying cabinets, despite their effectiveness, weren’t being widely used — less than half of endoscopy centers had them at that time.
Unfortunately, lingering moisture in endoscope channels can cause problems as microbes can reproduce and form in those conditions. Forced-air drying cabinets have proven effective combating that.
“If there are no microbes in the channels of an endoscope and it’s totally dry, that’s a pristine scope that can be used with confidence that it is not going to transmit exogenous microorganisms to the patient it is used on,” Alfa told G&E News.
Bedside Pre-Cleaning
Bedside pre-cleaning remains the most important step in reducing the risk of biofilm formation on flexible endoscopes, according to Alfa and Cotes. Plenty of research has chronicled the risk of not performing this step and showing how microorganisms can survive high-level disinfection (HLD) if protected by biofilm.
Sterilization vs. High-Level Disinfection
Afla and Cote agree that ethylene-oxide (EtO) sterilization provides very little extra protection against cross-contamination compared with high-level disinfection (HLD).
Plus, EtO sterilization is more expensive, harder to implement, and very few sites — especially clinics and ambulatory surgery centers — have access to it onsite. Facilities forced to send out endoscopes for sterilization could have additional infection prevention problems — conditions are ripe for bacterial replication and biofilm formation if an endoscope has moisture in the channel and travels for a few days before sterilization.
Low temperature sterilization, as compared to EtO sterilization, is cheaper and provides a faster turnaround time, according to the experts. Ultimately, the success of these processes depends largely on the pre-clean and manual clean.