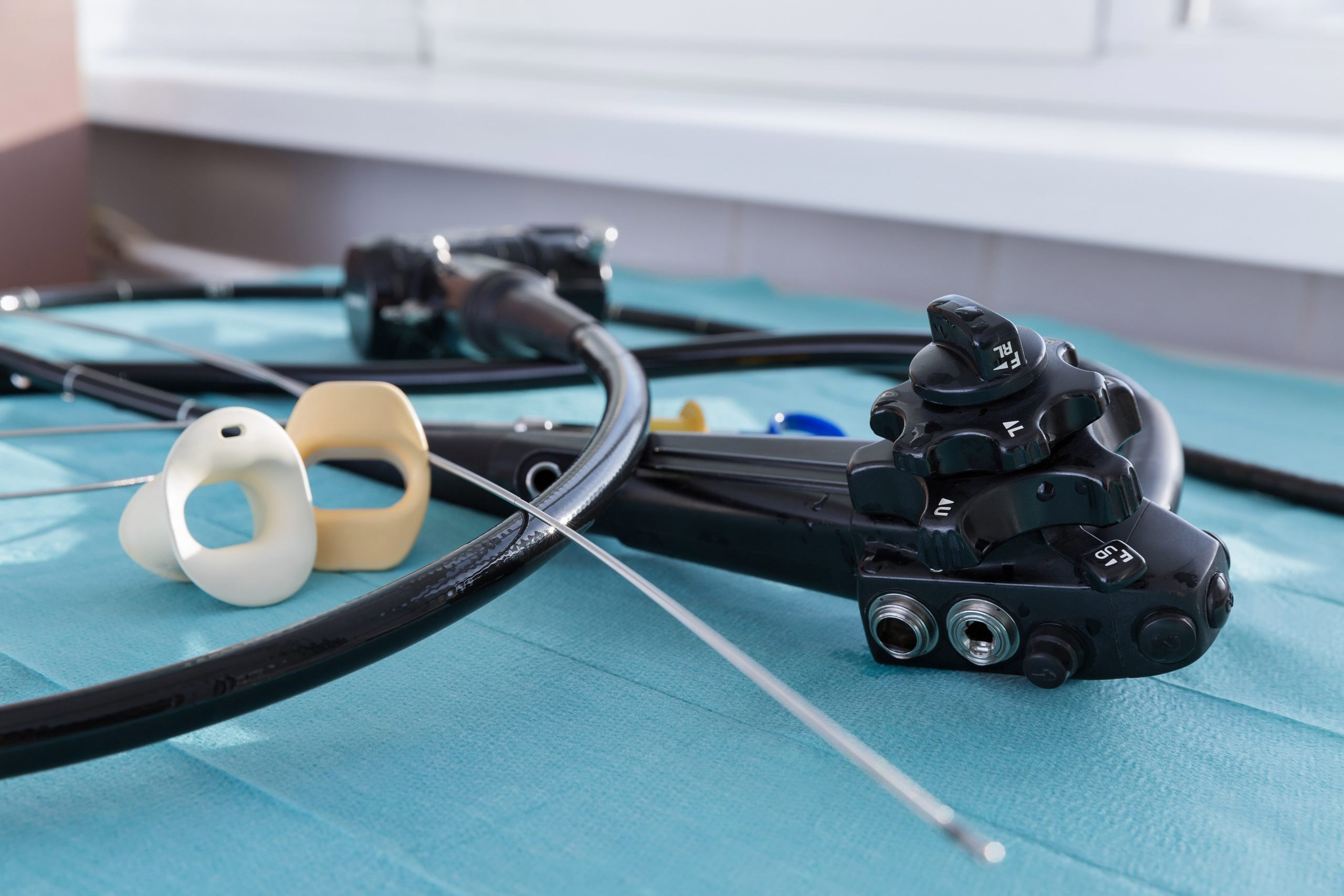
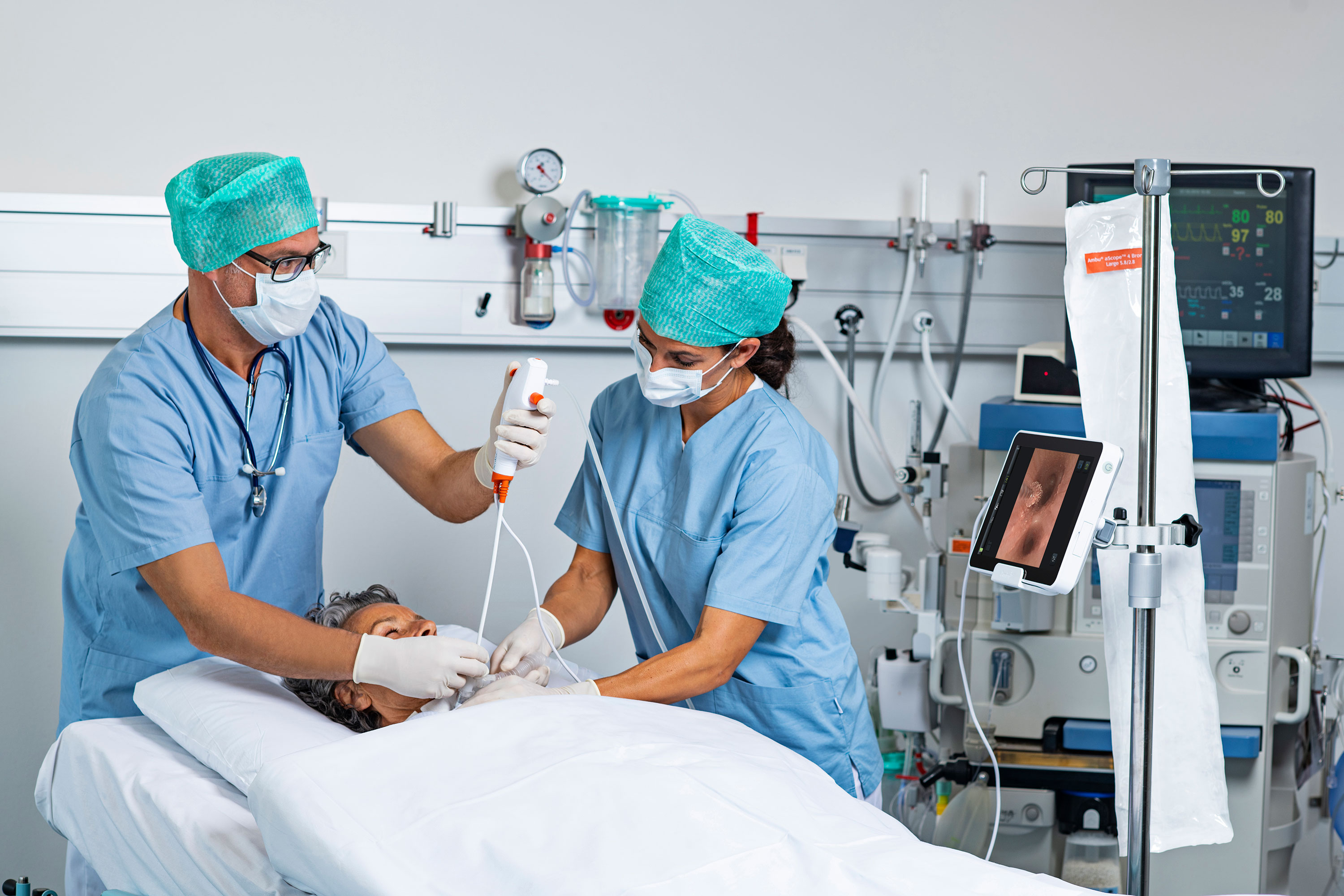
Now is an ideal time for urology departments and infection prevention teams to work more closely together on everything from risk points for potential infections to what types of endoscopes to purchase.
After all, urology is a high-volume specialty with high-risk surgeries performed using complex medical instruments. And yet it typically hasn’t been subject to the same level of infection surveillance scrutiny as other specialties and procedures.
That was one takeaway from a session on Day 2 of the Association for Professionals in Infection Control and Epidemiology (APIC ) annual conference, held in Indianapolis June 13-15. About 1,300 attendees trekked to the Hoosier State’s capital city for the in-person conference while another 1,800 were opting to take in the sessions and continuing education courses online, according to APIC officials.
The session on integrating urology into infection prevention programs was led by Jill Holdsworth, infection prevention manager for Emory University Hospital Midtown in Atlanta, and Dr. Adam Lorentz, a urologist at Emory University Hospital Midtown. Lorentz walked attendees through the diverse range of procedures urologists perform – and the many chances for infection.
He also poked fun at himself and his fellow urologists for wanting a cystoscope processed in 10 minutes or having their eyes glaze over at a ureteroscope manufacturer’s densely worded instructions for use. Clinicians are primarily focused on optimizing quality and efficiency.
“What the urologist cares about is that the scope is there when the patient is there,” Lorentz said.
Recent regulatory scrutiny makes the timing right for this kind of enhanced collaboration with IPs. In April 2021, the U.S. Food and Drug Administration said it was investigating “numerous” medical device reports (MDRs) describing patient infections and other possible contamination issues possibly associated with reprocessed urological endoscopes.
These endoscopes include cystoscopes, ureteroscopes, and cystourethroscopes.
Between Jan. 1, 2017, and Feb. 20, 2021, the FDA received more than 450 MDRs describing post-procedure patient infections or other potential contamination issues involving reprocessing these endoscopes.
Some reports have indicated issues with inadequate reprocessing or maintenance. Other potential issues the FDA is evaluating include device design and reprocessing instructions on labeling.
Earlier this year, a comprehensive review and analysis of adverse event reports involving flexible endoscopes filed with the U.S. Food and Drug Administration showed a marked increase for six types of scopes from 2014 through 2021. These include urological endoscopes.
Medical device manufacturers such as Boston Scientific Corp. and Ambu A/S have developed single-use endoscopes, including cystoscopes, touting their availability and ability to eliminate patient cross-contamination because they are used once and discarded. Lorentz said his practice is considering single-use scopes.
A formative step, Holdsworth said, could be something as simple as working together on what kinds of scopes to purchase – before they’re acquired only to frustrate urologists for being out of service for reprocessing or damage.
That in turn can lead to impactful initiatives such as targeting catheter-associated urinary tract infections, or CAUTI. Approximately 75 percent of UTIs acquired in a hospital are associated with a urinary catheter, and between 15 percent to 25 percent of patients receive urinary catheters during their hospital stay, according to the U.S. Centers for Disease Control and Prevention.
Day 1 of APIC’s annual conference explored the implications of the recently released and highly anticipated update to standards for reprocessing flexible endoscopes, as well as moving medical instrument sterilization offsite and the impact of COVID-19 on the field.
Founded in 1972, APIC counts more than 15,000 members advancing the science and practice of infection prevention and control through research, advocacy, and patient safety; education, credentialing, and certification; and fostering development of the infection prevention and control workforce of the future.