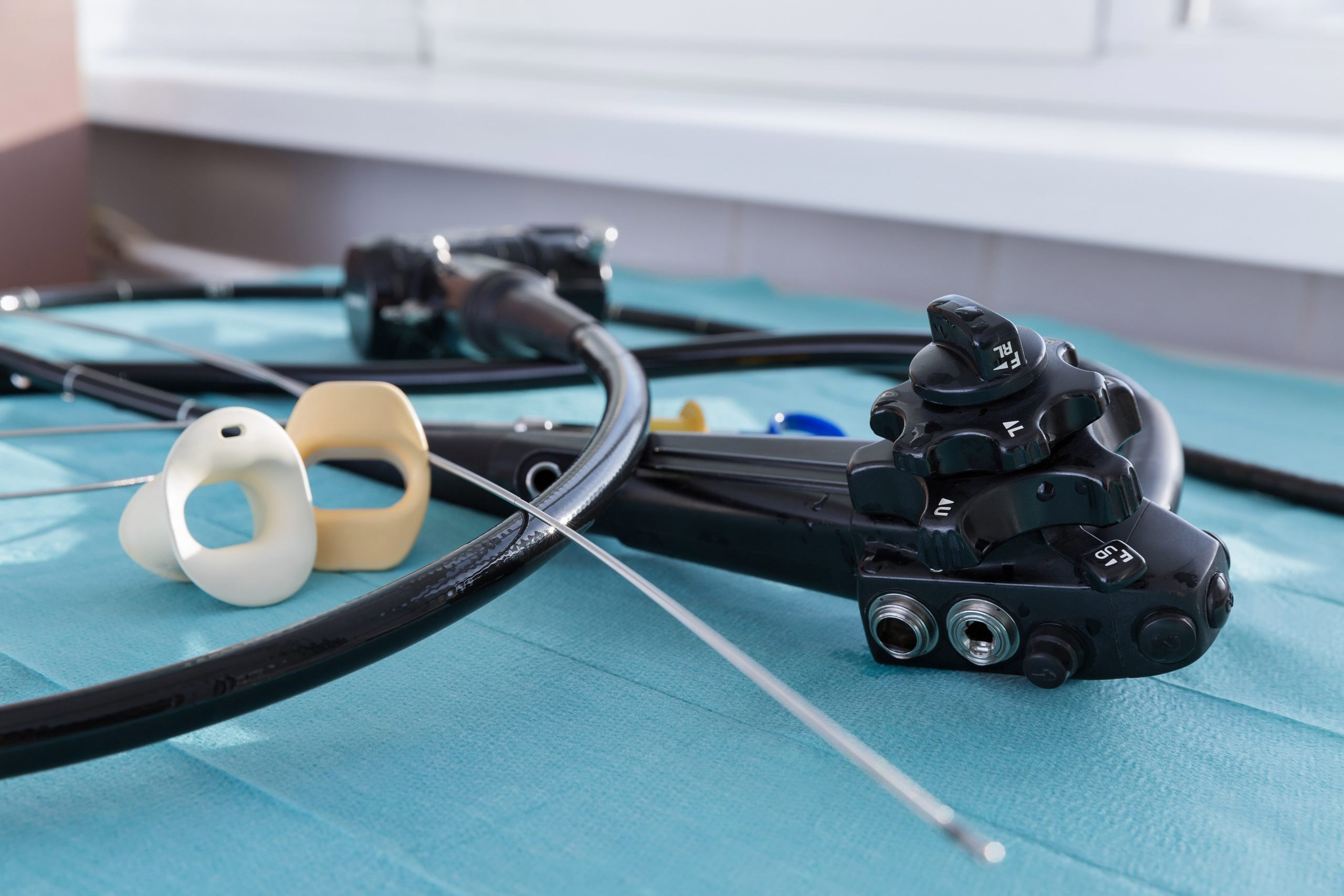
The U.S. Food and Drug Administration posted on its medical device database a Class 2 recall on single-use distal covers for duodenoscopes that was initiated by global healthcare company Olympus.
The recall was initiated by Olympus on April 18 and posted to an FDA database on May 19. Recall notification letters were sent to customers on April 23 instructing customers to “immediately locate and no longer use” the original design of model MAJ-2315.
“Correct application and inspection of the distal cover before the procedure is critical,” the letter also states. “In addition, inspection of the distal end after the procedure is also important.”
The recalled distal covers may cause mucosal injury, according to the database posting. The recalled product possesses the risk of becoming detached from the duodenoscopes during use, which may lead to the risk of aspiration or inhalation. Additionally, a detached endcap may cause obstruction requiring urgent removal or lead to burns caused by an uncovered distal end.
Earlier this year, the FDA issued its third warning letter in a six-month span to Olympus. It stated that the endoscope manufacturer received a 2020 complaint that "a patient sustained esophageal trauma" and "tissue from the esophagus was caught up in the distal tip of the device," which was associated with “cracked caps.”
Additionally, the letter stated that since November 2020 Olympus had received approximately 160 complaints describing cases where distal end covers “dropped out.” Olympus, according to the FDA, has failed to establish and maintain procedures for implementing corrective and preventive action.
“Olympus’ continued failure to meet FDA requirements demonstrates a troubling disregard for patient safety,” Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, said in a March news release.
An Olympus spokesperson issued a statement to the New York Times that same month saying that the company had moved to address the deficiencies outlined by the FDA but would “further evaluate these actions” and “expand those efforts.”