Recent studies showing post-endoscopic infection rates being more common than previously believed underscore the importance of a safe and systematic approach to reprocessing flexible endoscopes.
Facilities follow detailed guidelines for endoscope cleaning and disinfection. There’s less guidance, however, when it comes to storage. That means hospitals must weigh risk-mitigation questions against their facility’s unique operating constraints.
There are a variety of storage solutions available to address these needs. How to select among them? Here are four considerations you need to weigh with your storage solutions, as well as some features of three products on the market today.
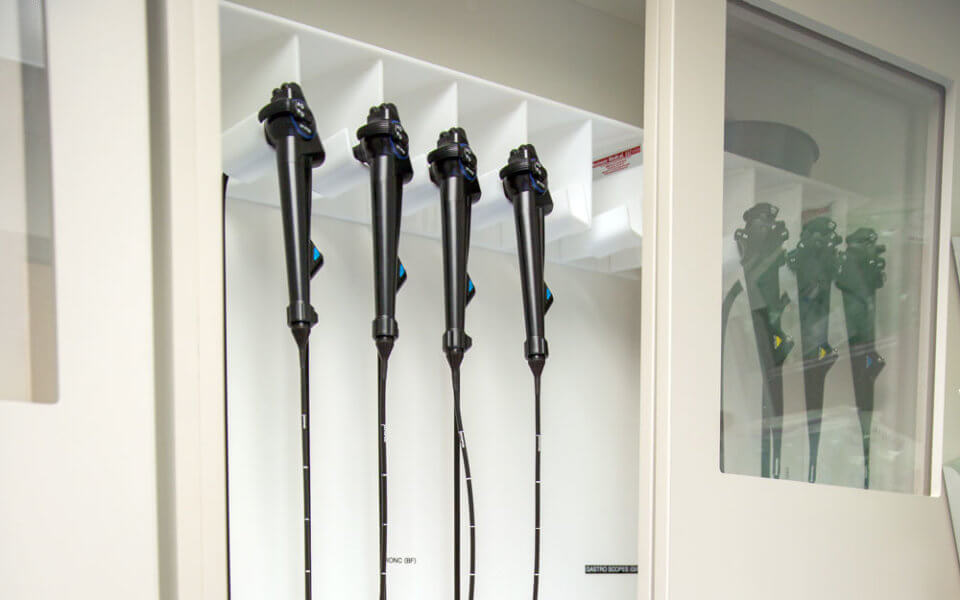
Advisory committees and oversight agencies generally recommend vertical storage. While in storage, instruments should not contact one another and their distal tips should hang freely.
To facilitate thorough drying and limit the risk for contamination, endoscopes should be stored in a well-ventilated area. The Association of periOperative Registered Nurses, or AORN, specifically calls for HEPA air filtration, and both The Joint Commission and the Society of Gastroenterology Nurses and Associates (SGNA) recommend dust-free environments.
Storage capacity typically ranges from six instruments to 20 or more, with optimal capacity depending on patient volumes. For smooth endoscope retrieval, AORN and The Joint Commission both recommend placing storage cabinets in a centralized—but unobtrusive—location outside of the procedure room. This makes size and footprint key factors to consider.
AORN and ANSI make additional recommendations for scope logging and retrieval. Scopes should be labeled with processing dates, the name of the processor and the date of high-level disinfection. Some storage solutions digitize this process.
The following products, all vertical drying cabinets available in the U.S., meet these guidelines. Weighing your needs through the prism of these products and their offerings can help inform your organization’s approach to reprocessing.
CleanShield®, from AirClean Systems
CleanShield is the most compact of the three, standing almost eight feet tall and a little more than three feet wide and, with a depth of about two feet. It holds a maximum nine units.
HEPA filtration provides continuous air flow with minimal particulate matter circulation. The cabinet’s door can be locked but the scopes are visible behind a clear viewing panel.
Installing a CleanShield requires no facility modifications—it’s plug and play using a standard electrical outlet. While it lacks some of the advanced security and inventory features offered by competitors, the CleanShield’s size and functionality make it a serviceable storage solution for outpatient care centers.
Olympus’s ScopeLocker is simple to set up and distinguishes itself with a few additional features.
For one, the ScopeLocker has broader capacity options. Facilities can choose four-, eight-, nine- or 18-scope cabinets, the largest of which stands almost eight feet tall and almost three-and-a-half feet wide, with a depth of a foot-and-a-half.
Its HEPA filtration replaces the cabinet’s air every two minutes and comes with a built-in fan to improve circulation.
The ScopeLocker also boasts a unique construction. Its rotating storage hooks allow access to rear-hanging scopes, without removing any scopes in the front, and the cabinet’s walls are padded, protecting distal ends from potential damage.
Optional accessories from Olympus—such as LED lights, hygrometers and keyless entry locks—can boost the ScopeLocker’s utility.
The Reliance™ 6000 Series, from Steris
The Reliance 6000-series cabinets come in six-, 10-, 16- or 20-scope capacity options. Additional cabinets can store up to 20 more endoscopes. The largest has dimensions of almost four feet wide and almost eight feet high, with a depth of two feet.
Its HEPA filtration is one of the most robust on the market, replacing the cabinet’s air every 20 seconds. And the 6000 series has additional features that should appeal to equipment managers—swipe-badge access restriction, bar-code scanning for inventory, built-in LED lights, automatic hang-time tracking and a removeable drip-pan floor to eliminate any possibility of standing water.
These features make the Reliance 6000 a good choice for high-volume facilities with a large endoscopy workforce.
Storage is just one part of endoscopy reprocessing. Like other components of healthcare systems, endoscopy storage should be considered in its larger context, with the ultimate goal foremost in mind—keeping patients safe from infection.